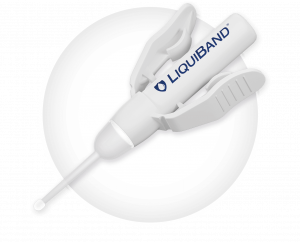
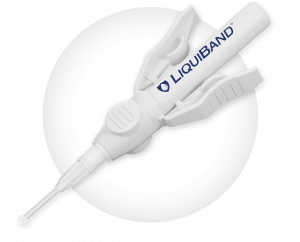
LiquiBand® Exceed™ XS
LiquiBand® Exceed™ XS offers the improved attributes of precision and controlled delivery with added advantages of an innovative tip design.
Description
Key Benefits
High Viscosity3
-
Provides optimal flexibility
Controlled expression of adhesive4
-
Applicator offers control during expression of adhesive
Microbial barrier5
-
Acts as a barrier against gram-positive, gram-negative and fungal microbes. Proven effectiveness against S. aureus, P.aeruginosa, E.coli, Candida albicans and MRSA
No Clog Formulation6
-
Allows users to work at their own pace
-
One applicator covers 7cm of wound length
Maintains wound closure throughout healing process7
-
The adhesive will stay on the skin for 5-10 days and sloughs off naturally
Giving protection against:
| Gram Positive Bacteria | Gram Negative Bacteria | Fungi |
|---|---|---|
| > S. Epidermidis | > Ps. Aeruginosa | > Asp. Brasiliensis (niger) |
| > S. Aureus & MRSA | > E. Coli | > C. Albicans |
| > E. Cloacae |
Design Features
Pure octyl formulation
-
Maximises durability and flexibility
-
Provides high viscosity
-
Creates intimate skin contact
Unique applicator tip
-
Elliptical shape allows for narrow or wide application
-
Porous felt tip allows drip-free priming and even, consistent application
Easy to use applicator
-
Winged applicator allows for safe and easy activation
-
Provides controlled delivery of adhesive
No clog technology
-
Tip will not clog, allowing consistent flow of adhesive
-
0.4mL of adhesive per applicator delivers approximately 7cm of coverage