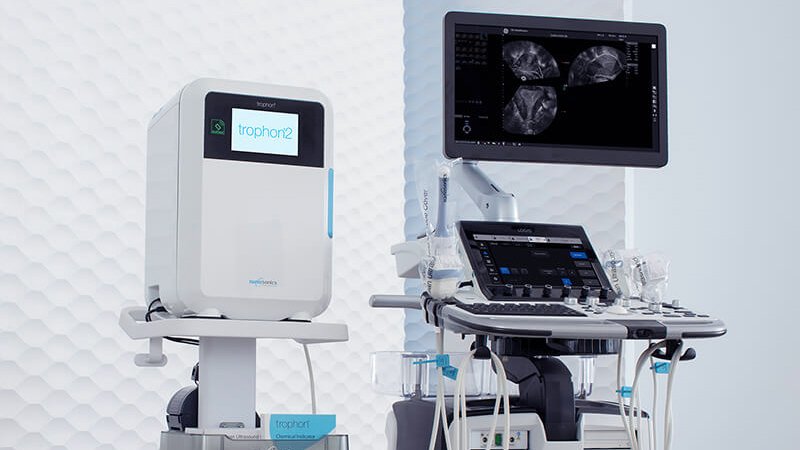
Ultrasonic probes disinfection - trophon®2
Advanced disinfection of ultrasound probes, right at the point of care
Efficient workflow at the point of care
The trophon®2 integrates seamlessly into the clinical workflow, enabling disinfection at the point of care. Thanks to its fully enclosed system and intuitive touchscreen interface, manual handling time is minimized. This makes the device ideal for use in busy outpatient clinics and primary care settings.
trophon®2 disinfection of ultrasound probes
Advanced microbial protection
The trophon®2 meets the stringent microbial efficacy standards required for both CE marking and FDA registration. The system eliminates a broad range of clinically relevant pathogens, including multi-drug resistant bacteria, blood-borne viruses, and sexually transmitted infectious agents.
With integrated AcuTrace® RFID technology, the trophon®2 automatically records all disinfection process data. This eliminates the need for manual logging, reduces errors, and supports audit readiness and protocol compliance.
Broad compatibility with ultrasound probes
In collaboration with probe manufacturers, Nanosonics has developed an industry-leading compatibility program. To date, over 1,300 probes from 26 manufacturers have been tested and approved for use with the trophon®2. This includes wireless probes, making the trophon®2 the first and only automated high-level disinfection (HLD) solution for wireless ultrasound probes.
Safe for users and the environment