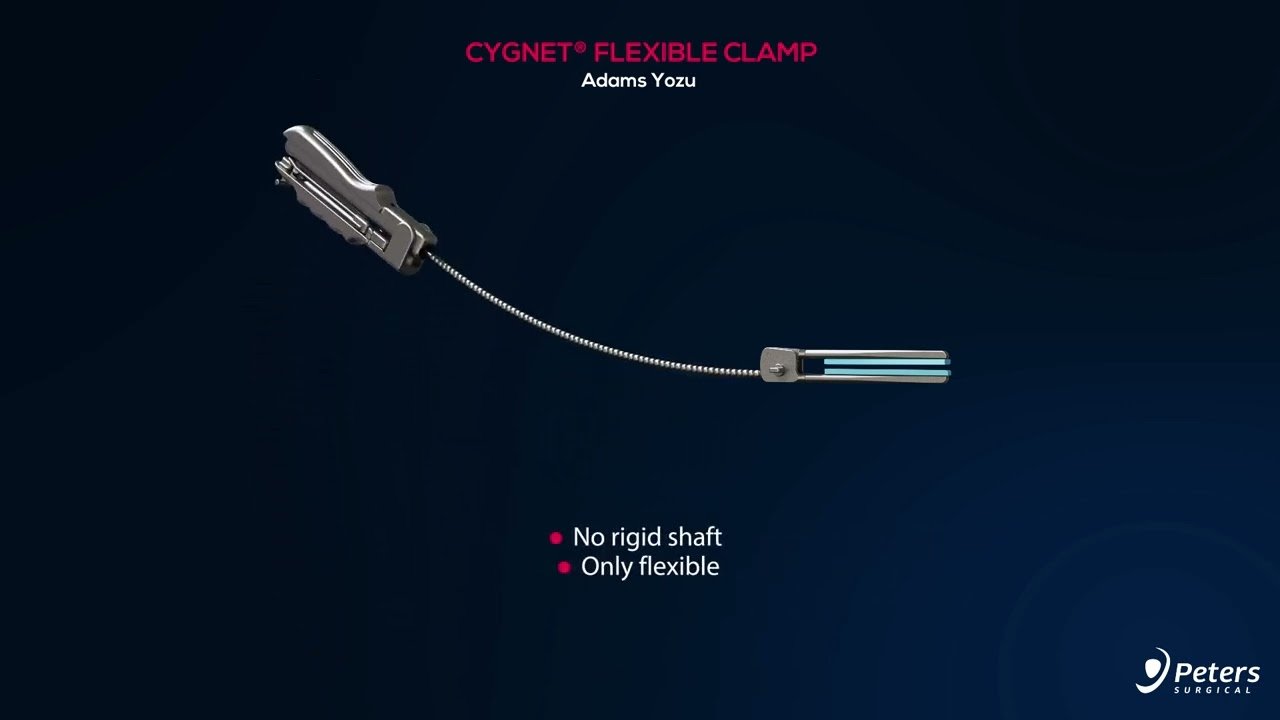
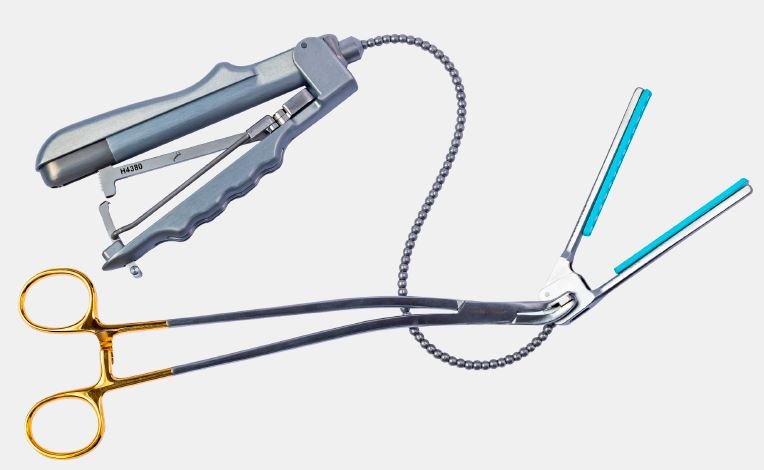
Fexible Clamps
Application: Minimally invasive cardiac surgery, CABG procedures, temporary occlusion of vessels / prostheses
Key Features
Clamps
| Feature | Description |
|---|---|
| Brand / Series Names | Cygnet®, Intrack® Inserts |
| Multi-use / Reusable | Yes — the Cygnet® Flexible Clamps are reusable. |
| Retractable / Low-profile Design | The rigid shaft enables tunnelling. Once positioned, the shaft retracts leaving a flexible neck, allowing better manoeuvrability around the surgical site. |
| Protection Compatibility | Intrack® Inserts are single-use, interchangeable, atraumatic, and compatible with both Cygnet® Flexible Clamps and Intrack® Clamps. |
| Variants / Models | • 28 clamp models (straight, angled or curved jaws) • 3 jaw lengths: 33 mm, 66 mm and 86 mm |
| Jaw Protection Options | 4 protection models, designed to be atraumatic to vessels. |
| Regulatory Classification | Cygnet® Flexible Clamps: Class I medical device (CE marked), manufacturer: Vitalitec Inc. Intrack® Inserts: Class IIb medical device, CE 2797 BSI. |
Specifications
- Jaw lengths: 33 mm, 66 mm, 86 mm
- Jaw shapes: Straight, curved, angled
- Material & Manufacturing: (If available: material types, finishes etc.)
- Regulatory status: CE-marked; clamps are Class I, inserts are Class IIb.
Usage & Precautions
- Intended for professional surgical use only; in hospitals / clinical settings.
- Carefully read the Instructions for Use before operating.
- Ensure inserts are compatible in shape & size with the clamp model.
- Follow prescribed sterilisation protocols.
Benefits