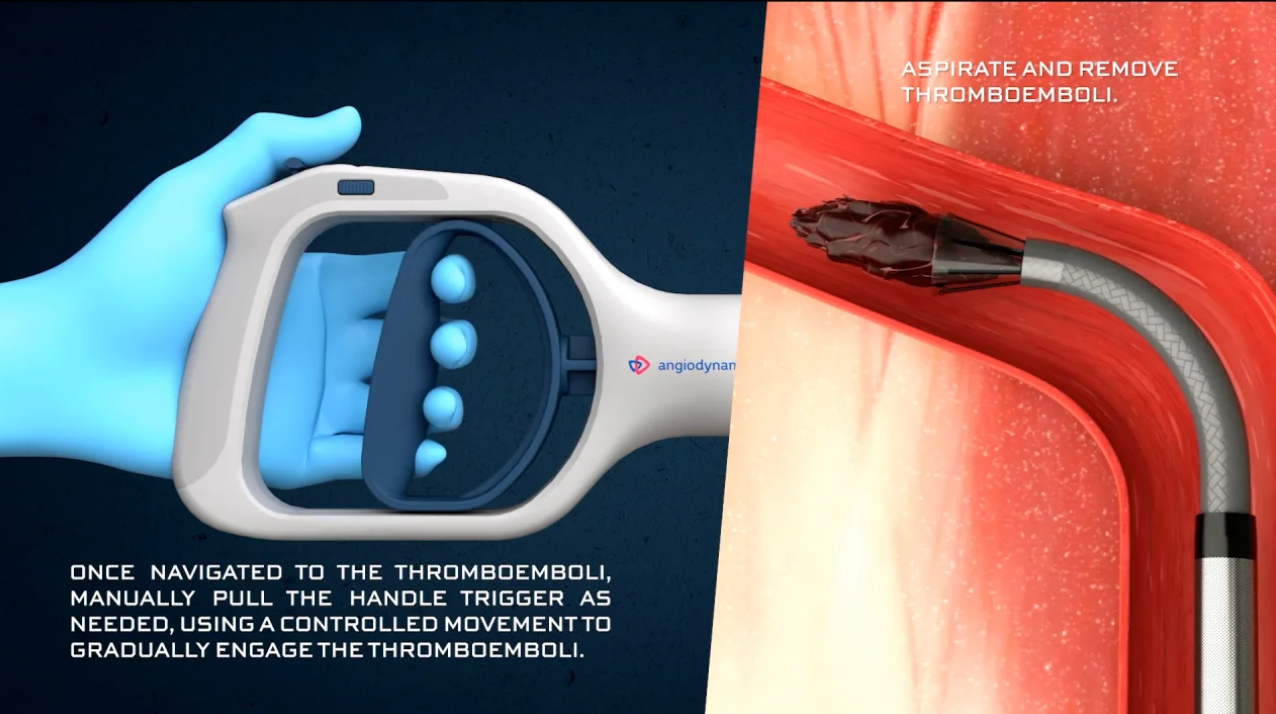
Mechanical Aspiration - AlphaVac™ F18⁸⁵ System
The AlphaVac F18⁸⁵ System by AngioDynamics is a user-friendly, percutaneous mechanical aspiration system designed for the non-surgical removal of thrombi or emboli from the venous system. Recently CE-certified in Europe and FDA-cleared in 2024 for the treatment of pulmonary embolism (PE).
Key Features & Benefits
Benefits and Clinical Evidence
- Ergonomic vacuum handle with volume lock (10 cc / 30 cc) and one-touch vacuum hold for safe and efficient aspiration
- 18 F funnel-shaped cannula with an 85° angled tip, 105 cm long – expandable to 33 F for optimized access to the pulmonary vasculature
- Continuous flow-through system – uninterrupted procedure even in critical care scenarios
- No perfusionist required – simplified setup for time-sensitive interventions
- Clear waste collection bag for visual confirmation of thrombus removal
- APEX‑AV Trial (USA):
122 patients with intermediate-risk pulmonary embolism (PE).
Average reduction in RV/LV ratio after 48 hours was –0.45 (p < 0.001).
Major adverse events occurred in 4.1% of patients (well below the <25% threshold). - Case Reports:
Showed positive outcomes, including improved right ventricular (RV) function, reduced pulmonary pressures, and rapid clot burden reduction (≈ 35–37%). - RECOVER‑AV Trial (Europe):
Ongoing multidisciplinary study evaluating mid- and long-term clinical outcomes.
First patient enrollment was recently announced.