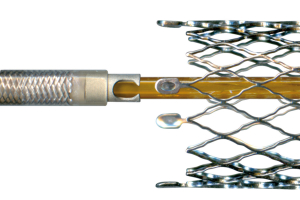
Stent for the prevention of carotid strokes: CGuard™ Embolic Prevention System (EPS)
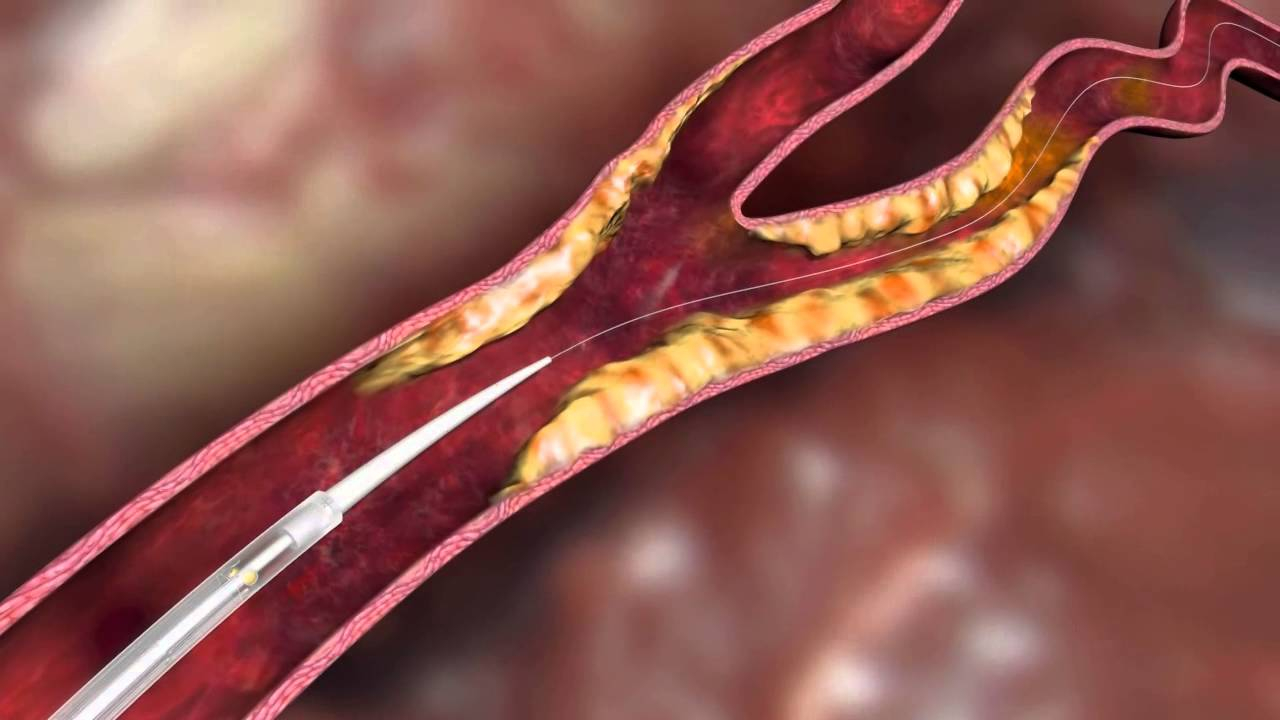
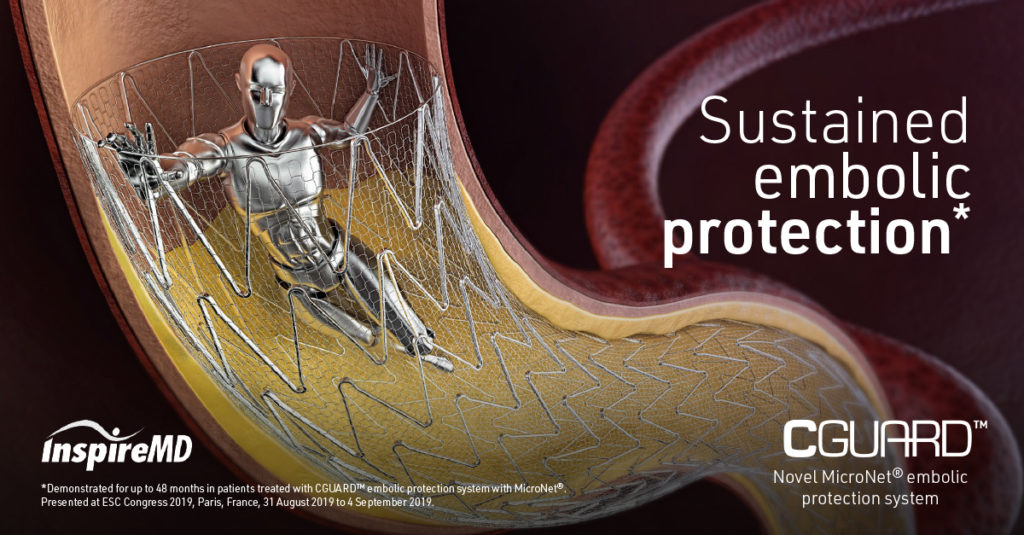
The CGuard™ Embolic Prevention System (EPS) is an innovative carotid stent designed to prevent strokes during the treatment of carotid artery stenosis. During carotid revascularization, small embolic particles can become dislodged and cause a cerebral infarction. CGuard™ actively prevents this with its MicroNet® technology, which securely holds plaque and thrombus against the vessel wall.
The stent combines open-cell flexibility with closed-cell protection, ensuring optimal fit, visibility, and placement accuracy.
Thanks to the user-friendly 6F rapid exchange delivery system, physicians can easily and precisely position the stent, even in complex anatomies.
Description
Key benefits
Continuous embolic protection: The MicroNet® sleeve features the smallest pore size on the market (150–180 µm) and effectively prevents embolic material from escaping, both during and after the procedure.
SmartFit™ technology: Automatically adapts to the vessel diameter. A tapered design is not required, ensuring optimal placement and coverage.
Proven clinical performance: Studies show a restenosis rate of less than 1% after 12 months¹, confirming the system’s effectiveness and durability.
Excellent visibility and precision: The system is highly visible under all imaging modalities, allowing for extremely accurate positioning.
Flexible and safe deployment: The self-expanding nitinol stent combines exceptional flexibility with minimal shortening and safe post-dilatation.
Clinical Evidence
IRON-Guard Study (EuroIntervention, 2018)
Demonstrated a restenosis rate of less than 1% after 12 months¹.
PARADIGM Study (EuroIntervention, 2020)
Confirmed the safety and effectiveness of CGuard™ EPS in both symptomatic and high-risk asymptomatic patients².
Summary
- Continuous embolic protection with MicroNet® technology
- SmartFit™ design for optimal vessel conformity
- Less than 1% restenosis after 12 months
- Excellent visibility and placement accuracy
- Self-expanding nitinol design offering high flexibility
Technical specifications
| Feature | Description |
|---|---|
| Stent material | Nitinol |
| MicroNet® material | PET (Polyethylene Terephthalate) |
| Fiber thickness | 23 µm |
| Pore size | 150–180 µm |
| Strut thickness | 240 µm ±15 µm |
| Delivery system | 6F Rapid Exchange |
| Catheter length | 135 cm |
| Guidewire compatibility | 0.014” |
| Sheath compatibility | 6F (ID > 2.20 mm) |
| Available diameters | 6, 7, 8, 9, 10 mm |
| Available lengths | 20, 30, 40, 60 mm |
Technical Services