Mechanical aspiration – AlphaVac™ F18⁸⁵ System
The AlphaVac™ F18⁸⁵ System from AngioDynamics is a user-friendly, percutaneous mechanical aspiration system specifically designed for the non-surgical removal of thrombi or emboli from the venous circulation. It was recently CE-certified in Europe and received FDA approval in 2024 for the treatment of pulmonary embolism (PE).
Features & Benefits
Advantages and clinical evidence
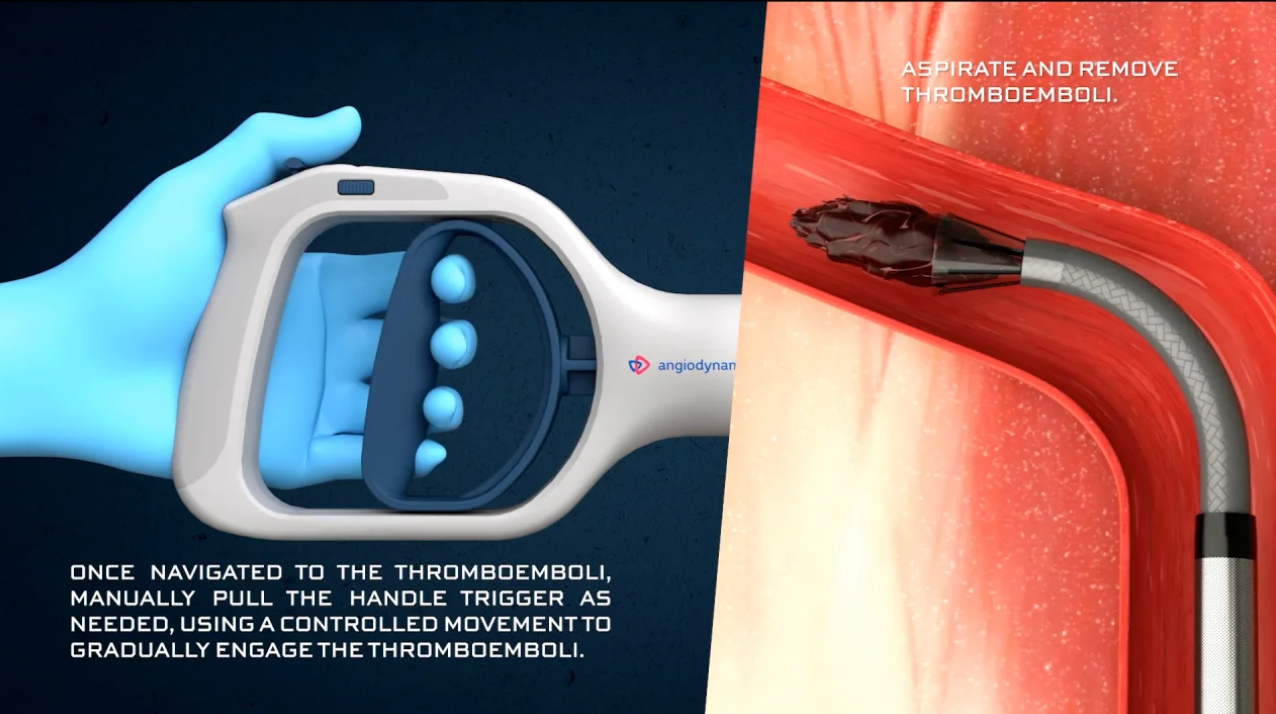
- Ergonomic, steady handle with volume limitation (10 cc / 30 cc) and vacuum lock for improved control and reduced blood loss
- 18 F funnel-shaped cannula with an 85° angle, 105 cm length – expandable up to 33 F, designed for optimal navigation within the pulmonary anatomy
- Single, continuous flow-through process without interruption, ensuring efficiency in urgent situations
- No perfusionist support required – simplified setup, suitable for emergency use
- Transparent waste bag allows visual confirmation of aspirated thrombus
- APEX-AV trial (US): 122 patients with intermediate-risk pulmonary embolism (PE). Mean reduction in RV/LV ratio at 48 hours was −0.45 (p < 0.001); major adverse events: 4.1% (below the 25% benchmark).
- Case reports: Positive outcomes observed in right ventricular (RV) function, decreasing pulmonary pressures, and rapid reduction of clot burden (≈ 35–37%).
- RECOVER-AV trial (Europe): Multidisciplinary study evaluating mid- and long-term outcomes; first patient enrollment recently reported.
Documents